Mounjaro Approved for Sleep Apnea Treatment: How the Weight Loss Drug Works
Australia has approved the use of Mounjaro, a weight-loss drug, for treating sleep apnea in obese adults. Learn how this medication works and its potential benefits.
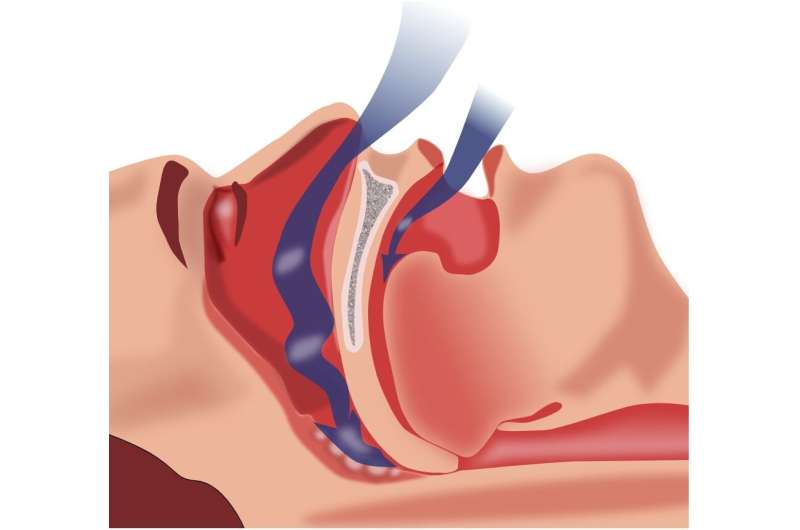
Last week, Australia's Therapeutic Goods Administration (TGA) granted approval for Mounjaro, a medication primarily used for weight management, to be utilized in treating sleep apnea in adults with obesity. Sleep apnea, a common sleep disorder affecting roughly one billion people globally, involves repeated episodes of airway obstruction during sleep, leading to interruptions in breathing. These disruptions can cause fragmented sleep, decrease oxygen levels, and increase the risk of serious health issues such as cardiovascular disease, diabetes, hypertension, and stroke.
Obesity is a significant risk factor for sleep apnea, with about 80% of affected individuals being obese. Excess fat around the neck narrows the airway, making it more susceptible to collapse during sleep. Conversely, sleep apnea can worsen weight gain by disrupting hormones that regulate hunger and fullness, creating a vicious cycle.
Traditionally, treatment has involved the use of Continuous Positive Airway Pressure (CPAP) machines, which keep the airway open during sleep by delivering pressurized air. Despite their effectiveness, many patients find CPAP devices uncomfortable, which can hinder consistent use. Therefore, new treatment options are in high demand.
Mounjaro, also known as tirzepatide, is a drug that mimics two gut hormones—GLP-1 and GIP—which regulate appetite, blood sugar, and food intake. By activating these hormone receptors, tirzepatide helps people feel fuller sooner, typically resulting in reduced food consumption and weight loss. In a study involving 469 individuals with obesity and moderate to severe sleep apnea, a year of tirzepatide treatment led to up to a 60% reduction in sleep apnea severity, compared to a mere 3% decrease with placebo.
Beyond weight reduction, tirzepatide is associated with improvements in key health indicators, such as reduced inflammation, better insulin sensitivity, and lower blood pressure. These benefits could enhance respiratory function and help prevent cardiovascular and metabolic complications linked to sleep apnea.
However, Mounjaro can cause side effects like nausea, vomiting, diarrhea, constipation, and loss of appetite, which usually lessen over time. Gallbladder problems have also been reported by some patients. It is important to note that the drug is approved for individuals with obesity, and not all sleep apnea patients will be candidates, especially those without excess weight or specific airway anatomy contributing to their condition.
In Australia, Mounjaro remains a private prescription medication costing approximately A$395 per month, as it is not subsidized under the Pharmaceutical Benefits Scheme. This substantial cost may limit access for many patients.
While the approval marks a promising advancement in sleep apnea management, further studies are needed to determine its long-term efficacy and safety across diverse patient populations. Currently, pharmacological options like Mounjaro could complement existing treatments, especially for those struggling with compliance to device-based therapies.
Stay Updated with Mia's Feed
Get the latest health & wellness insights delivered straight to your inbox.
Related Articles
Low-Calorie Diet May Not Reduce Hip Osteoarthritis Pain but Offers Other Health Benefits
A low-calorie diet combined with exercise does not directly reduce hip osteoarthritis pain but can improve physical function and promote weight loss, offering additional health benefits for affected adults.
RFK Jr. Withdraws $500 Million Funding for Vaccine Development Initiatives
U.S. Health Secretary Robert F. Kennedy Jr. has announced the cancellation of 22 vaccine projects totaling $500 million, shifting focus to alternative vaccine strategies to fight respiratory viruses. This decision has sparked debate among health experts about the future of vaccine technology.



