Advanced Imaging Techniques Reveal How Weight Loss Medications Target the Brain and Pancreas

Innovative imaging techniques have provided new insights into how dual-agonist weight loss drugs like tirzepatide target cells in the brain and pancreas, paving the way for improved treatments for obesity and diabetes.
A collaborative international research effort involving the Leibniz-FMP, University of Oxford, and University of Birmingham has introduced a groundbreaking imaging method to understand how dual-agonist weight loss drugs like tirzepatide interact with cells in the pancreas and brain. Published in Nature Metabolism, this research enhances our grasp of the mechanisms behind these medications, potentially guiding the development of more effective treatments for obesity and diabetes.
The scientists engineered fluorescently tagged versions of molecules that mimic the effects of tirzepatide, a drug that activates two crucial hormone receptors—GLP-1R and GIPR. These molecules, called daLUXendins, enable real-time visualization of how the drugs move through the body and bind to specific cell types. This approach overcomes previous limitations where antibody-based methods were unreliable or unavailable for tracking receptor activity.
Tirzepatide, marketed as Mounjaro or Zepbound, is part of a new class of dual agonists that stimulate both GLP-1R and GIPR, receptors located in the pancreas—which regulate insulin—and in the brain, where they influence appetite. By mapping these pathways, the team uncovered how these drugs target cells both in the pancreatic islets and brain regions responsible for hunger regulation.
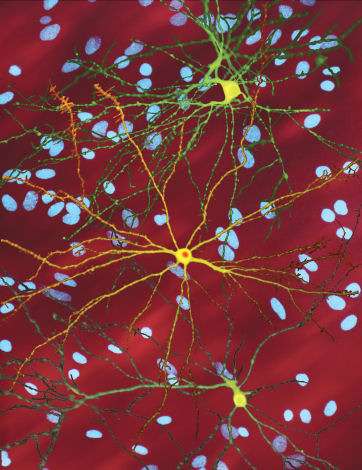
Using super-resolution microscopy, the researchers found that the fluorescent probes bind strongly to pancreatic beta cells, as well as alpha and delta cells, providing insight into their broad metabolic influence. In the brain, the probes revealed tirzepatide reaching areas involved in appetite control, including tanycytes that relay nutrient signals. Additionally, the study discovered that receptors cluster in tiny 'nanodomains' within the pancreas, which may help explain how dual-acting drugs amplify their signals.
While the current work was conducted using mouse models and fluorescent surrogates, the researchers believe their methods can be adapted for human studies and expanded to explore other therapeutic targets, including triple agonists that also act on glucagon receptors. This research sheds light on why dual agonists are so effective, while also prompting questions about their access to the brain and the potential of next-generation triple-acting drugs.
Professor David Hodson emphasized that future research will focus on comparing these findings with triple agonists, which might offer even greater benefits, advancing the development of more precise and powerful obesity and diabetes treatments.
Stay Updated with Mia's Feed
Get the latest health & wellness insights delivered straight to your inbox.
Related Articles
UK Researchers Achieve Breakthrough in Slowing Huntington's Disease Progression with Gene Therapy
UK scientists have achieved a historic milestone by slowing Huntington's disease progression through an innovative gene therapy, offering hope for future treatments and patient stability.
Innovative Algorithm Speeds Up Cell Identification to Improve Cancer Treatment Selection
A new AI-driven algorithm called TACIT can identify cell types within tissues in just minutes, revolutionizing cancer diagnostics and personalized treatment planning by providing fast, accurate cellular analysis.
Novel Mechanism Discovered in Cellular Resistance to Anticancer Drugs
Researchers have uncovered a novel DNA repair pathway involving Fen1 that enables cells to resist the effects of anticancer drugs like alovudine, opening new avenues for cancer treatment and biomarkers.
Gut Microbiota and Its Role in Autism Spectrum Disorder Progression in Mice
New research links gut microbiota to the progression of autism spectrum disorder (ASD) in mice, highlighting the potential for microbiome-based therapies to manage ASD symptoms through immune and neurotransmitter regulation.